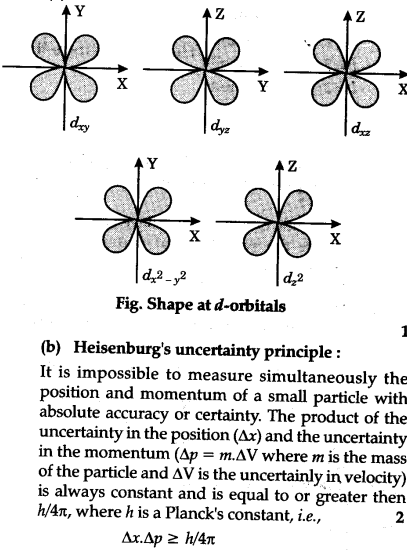
The arrow shows a qualitative representation of increasing orbital energy. D _ xy It is of cloverleaf-like shape.

Draw The Shapes Of Five D Orbitals
For an f orbital see below.

. Drawing 3-D Orbital StructuresDiagrams - KEY H H H H both Cs sp2 H H H H σ π double bond unhybridized p orbitals on Cs overlap above and below plane of molecule to form π bond f. If its 2 your atom is sp. Shape of d-orbitals.
It is best to assume this Web page has an affiliate romance andor A further material link for the persons or firms pointed out in or associated with from this page and should obtain commissions from purchases you make on subsequent Web pages. The energy of all five d orbitals. D orbital has two.
It is known to have doughnut shaped electron cloud around for the orbital d z 2 which is symmetrical around Z - axis. Hybridization in Phosphorus pentachloride PCl 5 sp 3 d 2 Hybridization. This video is a discussion about the shapes of atomic orbitals and the scientific principles that govern the shapes of atomic orbitalsThanks for watching.
To remember the shapes of orbitals follow the following. D _ yz It is of cloverleaf-like shape. Along the right side of the energy arrow write.
So depending upon the axes along which or between which the electron clouds are obtained different names and shapes are given as. The fifth d orbital is shaped like an elongated dumbbell with a doughnut around its middle. Steps for Drawing an Orbital Diagram Label the arrow energy.
It means d- orbitals can have five orientations. Click the images to see the various 3d orbitals There are a total of five d orbitals and each orbital can hold two electrons. Shapes and Energies of Atomic Orbitals Draw the structure of p - orbitals.
Count the number of atoms connected to it atoms not bonds Count the number of lone pairs attached to it. If its 4 your atom is sp 3. They are as follows.
Each orbital has four lobes. 3d xy 3d xz and 3d yz. Draw the shapes of the 1s 2s 2p x.
S l0 orbital has no nodal plane ie. Add these two numbers togetherAdd these two numbers together. D _ xz It is of cloverleaf-like shape.
For d-orbitals or d-subshell Ɩ 2 there are five values of m namely -2 -1 0 1 2. To make sense of the names we need to look at them in two groups. For example 3d xy 3d yz 3d zx 3d x2-y2 and 3d z2.
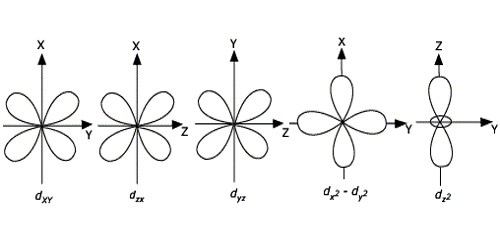
The d orbitals are given the designations d xy d yz d xz d x 2-y 2 and d z 2. How To Draw Shapes Of D Orbitals. At the third level there are a total of nine orbitals altogether.
The remaining two orbitals lie in the vertical plane at 90 degrees plane of the equatorial orbitals known as axial orbitals. No plane where electron density can be zero. For an s orbital draw a circle.
We know that the orbitals s p d and f have l azimuthal number number of angular nodes or nodal planes. Thus there exist five d orbitals. The magnetic orbital quantum number for d orbitals is denoted as -2 -1 0 1 2.
These are represented by d xy d yz d zx d x2-y2 and d z2. Thus d orbital corresponds to 4 double dumb-belled shapes d xy d yz d zx d x 2 y 2 with the atomic nucleus at its centre and one dumb belled with dough nut shaped d z 2. For d orbital Azimuthal quantum number l 2 and the magnetic quantum number m -2 -1 0 1 2.
The values for the d type orbitals are 2 1 0 -1 and -2. How to draw shapes of d orbitals Content Link DISCLOSURE. Draw the structure of p-orbitals.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. The d-orbitals have different shapes and these are only available when principal quantum number n 3 or more. Write out the electron configuration to determine which orbitals are filled.
When n 3 l 2 then m 2 1 0 1 and 2. Individual shapes of each d orbital. For example 3d xy 3d yz 3d zx 3d x 2-y 2 and 3d z 2.
In two dimensions we draw it as a circle. 3d x 2 - y 2. The five d-orbitals are designated as d x y d y z d x z d x 2 y 2 and d z 2.
They have an even more complex angular distribution than the p orbitals. The orbitals in an. Describe the shapes of s and p-orbitals.
The value of I 2 for d orbitals and for I 2 the five values of m are permissible. Draw the shape of all d orbirals. P orbital has 3 orientations.
It means five types of shapes of d-orbital in three-dimensional space. There are five 3d orbitals called. For a d orbital draw a four-leafed clover.
The d xy d yz and d zx orbitals have same shape ie clover leaf shape but they lie in XY YZ and ZX-. The shapes of the 3d orbitals. That means five d-orbitals are available in an atom.
Means d- orbitals can have five orientations. Hence d orbitals have five orientations in space. M l 0 1 2.
For a p orbital draw a figure eight. To make sense of it we need to look at these in two groups. D x 2-y 2 shape can be imagined as that of clover leaf with the respective two leaves each directed along X and Y axis.
The directions names and the shapes of these orbitals are as follows. These are represented by d xy d yz d zx d x 2-y 2 and d z 2. An illustration of the shape of the 3d orbitals.
Choose the correct statement from the given options. The five 3d orbitals are called 3dxy 3dxz 3dyz 3dx² - y² 3dz². Carbon dioxide CO2 O O sp sp2 O O lone pairs in sp2 orbitals 2 π bonds perpendicular to each other due to overlap of unhybridized p orbitals between C and.
Sp 3 d 2 hybridization has 1s 3p and 2d orbitals that undergo intermixing to form 6 identical sp 3 d 2 hybrid orbitals. Of the four s and p orbitals are considered because these orbitals are the most common in organic and biological chemistry. It is necessary to have the knowledge of the 3 d orbitals as it will be helpful in discussing the chemistry of many elementsthe 3d orbitals can be classified into 2 categories.
The shapes of the first four d orbitals are similar to one another while being different from the dz2 orbital. The transition metal series is defined by the progressive filling of the 3d orbitalsThese five orbitals have the following m l values. If its 3 your atom is sp 2.
The names tell you that these orbitals lie in the x-y plane the x-z plane and the y-z plane respectively. For d-subshell l 2 there are five values of m namely -2 -1 0 1 2. At the third level there is a set of five d orbitals with complicated shapes and names as well as the 3s and 3p orbitals 3px 3py 3pz.
An s orbital is a sphere. An s-orbital is spherical with the nucleus at its centre a p-orbitals is dumbbell-shaped and four of the five d orbitals are cloverleaf shaped.
I Draw The Shapes Of D Orbitals Sarthaks Econnect Largest Online Education Community
Draw The Shapes Of D Orbitals Heisenberg S Uncertainty Principle Sarthaks Econnect Largest Online Education Community

Draw The Shapes Of Five D Orbitals
Draw The Shapes Of D Orbitals Cbse Class 11 Chemistry Learn Cbse Forum

Draw The Shapes Of Various P And D Orbitals Youtube

Draw The Structure Of D Orbital

0 comments
Post a Comment